| |
|
Introduction to Structure Determination |
|
| NMR: The Spectrum |
|
|
|
|
|
|
A NMR spectrum contains lots of information about the functional groups and connectivity in a molecule. Whether you are predicting what the NMR spectrum of a compound will look like or trying to determine the structure of a compound from its NMR spectrum, a number of steps must be followed:
-
Use the chemical shifts and charateristic chemical shift tables (13C NMR) to determine the functional groups present.
-
Use the number of chemical shifts and the chemical formula (if available) to work out the number of equivalent nuclei and hence the presence or absence of symmetry in the molecule. For example, if there are 4 C atoms in the formula but only 2 chemical shifts in the 13C NMR spectrum then the molecule must possess symmetry which makes some of the nuclei equivalent.
Example 1: Predicting the Spectrum from a Structure
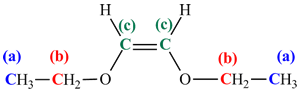
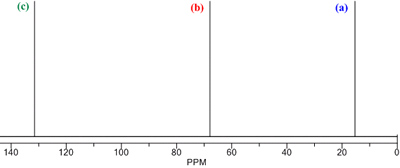
The process of predicting the spectra from a known structure is best illustrated with an example. The 13C NMR of the molecule to the right is shown on the spectrum below it. It can be rationalized using the steps outlined above:
-
Using the 13C NMR chemical shift table, the 13C chemical shifts are predicted to be:
(a) CH3 bonded to C: chemical shift 10 - 40 ppm (observed at 15 ppm).
(b) CH2 bonded to O: chemical shift 50 - 70 ppm (observed at 68 ppm).
(c) sp2 C atom in a C=C group: chemical shift 110 - 140 ppm (observed at 132 ppm).
- There is a mirror plane that cuts through the C=C bond. There are 3 chemical environments, (a), (b) and (c), and so 3 signals in the spectrum.
|
 |
|
Example 2: Predicting the Structure from a Spectrum
|
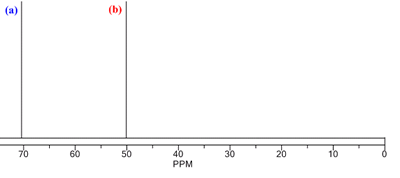
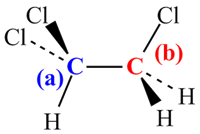
The process of predicting the structure from a NMR spectrum can also be best illustrated with an example. The 13C NMR of one of the isomers of trichlorethane, C2H3Cl3 is shown below. The structure of the isomer can be deduced using the steps outlined above:
|
-
Using the 13C NMR chemical shift table, the chemical shifts corresponding to (a) and (b) are both too high to be for a 13C nucleus in alkyl groups.
The 13C nuclei must all be bonded to at least 1 Cl atom. This rules out the isomer CH3CCl3. The chemical shift for (b) is consistent with a 13C nucleus in a RCH2Cl group. The chemical shift for (a) is higher and this is consistent with it being due to a 13C nucleus in a -CCl2R group, with the higher shift due to the additional electronegative Cl group.
- There are 2 C in the chemical formula and 2 peaks in the spectrum. The 13C nuclei are not equivalent.
All of the information is consistent with the structure opposite: 1,1,2-trichloroethane.
|
|
 |
| |
|
| |
|
 |
| |
| |




