Radiation in the infrared (IR) region of the electromagnetic spectrum has the energy to excite
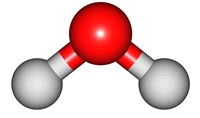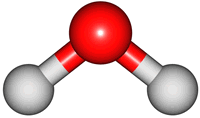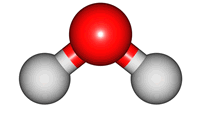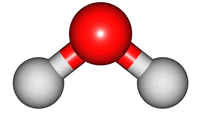
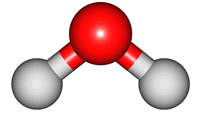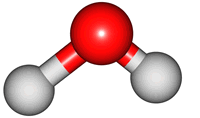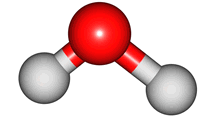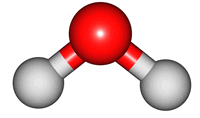
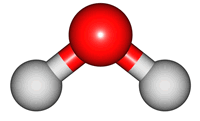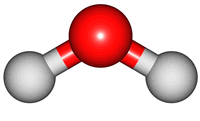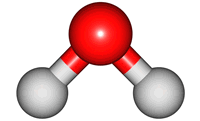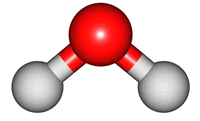
vibrations of covalent bonds. The absorption of IR radiation causes bonds to stretch and bend. Stretches correspond to the increasing and decreasing of the bondlenghs within a molecule. Bends correspond to the increasing and decreasing of the angle between bonds in a molecule. The animations below show the possible vibrations of the H
2O molecule. There are 3 vibrations: two stretches and 1 bend. 2 stretches are possible as the 2 O-H bonds can vibrate in sync ("symmetric") or out of sync ("asymmetric"). All 3 vibrations require different amounts of energy so the water molecule absorbs IR radiation at
3 different wavelengths.
| O-H stretches | Bend |
 |
 |
 |
| symmetric |
asymmetric |
bend |
The wavelength of the IR radiation that corresponds to the 2 stretches are quite similar. The bend takes less energy and it corresponds to a longer wavelength of IR radiation.
When IR radiation is shone on a molecule, it can only absorb it if:
- The energy of the radiation corresponds to the energy required to vibrate the molecule, and
- The vibration leads to the dipole moment of the molecule changing.
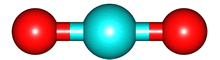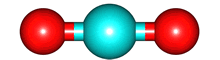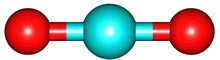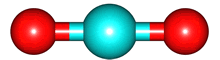
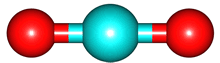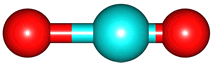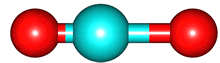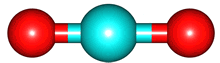
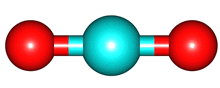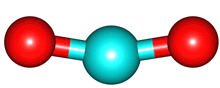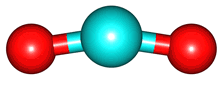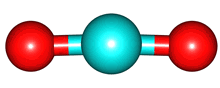
The vibrations of the CO
2 molecule are shown below. As for H
2O, there are 3 vibrations: 2 stretches and 1 bend. Although each C=O bond in CO
2 is polar, its linear shape means that the molecule as a whole does not possess a dipole moment. During the asymmetric stretch and bend, the symmetry is lost and a dipole is momentarily created. It is possible to excite these 2 vibrations with IR radiation. During the symmetric stretch, however, no dipole moment is created and so IR radiation is unable to excite this vibration. CO
2 thus absorbs at only
2 wavelengths in the IR. The difference between the number of absorptions in the IR spectrum of H
2O and CO
2 is a consequence of the difference in their shapes (bent and linear respectively). One use of IR spectroscopy is to help determine shape. Organic molecules, however, tend to be large and unsymmetrical and most vibrations change the dipole moment and can be excited by IR radiation.
| C=O stretches | Bend |
 |
 |
 |
| symmetric |
assymmetric |
bend |
In big molecules, there are many bonds and so a large number of possible stretches and bends are possible. The IR spectrum can be quite complicated. However, it is useful for two reasons:
- The IR spectrum can be used to identify the presence or absence of many organic functional groups and
- The IR spectrum of a molecule is unique. Just as a criminal can be caught by comparing his or her fingerprint to those in a database, a compound can be identified by comparing its IR spectrum with those in a library
.