| Neighbours |
Peaks |
Multiplicity |
Pattern |
| 0 |
1 |
singlet |
1 |
| 1 |
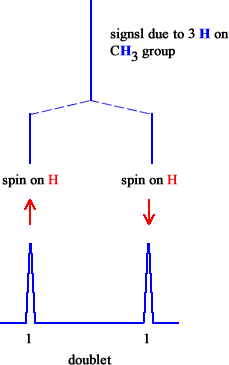
2 |
doublet |
1 : 1 |
| 2 |
3 |
triplet |
1 : 2 : 1 |
| 3 |
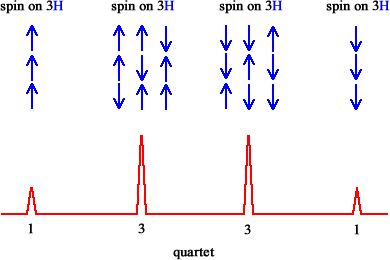
4 |
quartet |
1 : 3 : 3 : 1 |
| 4 |
5 |
quintet |
1 : 4 : 6 : 4 : 1 |
| 5 |
6 |
sextet |
1 : 5 : 10 : 10: 5 : 1 |
| 6 |
7 |
septet |
1 : 6 : 15 : 20 : 15: 6 : 1 |
Coupling between equivalent nuclei
No coupling is observed between nuclei which are equivalent. For example, the 3
H on the CH
3 in ethanol are all equivalent and no coupling
between these nuclei is observed. Only the coupling of the
H nuclei to the
H nucleus (and vice versa) is observed.
Effects of 13C and 13C NMR
In a
1H NMR spectrum, coupling to the
13C nuclei is not usually observed. Because of the low abundance of
13C, the chance of a
1H nucleus being next to a
13C nucleus is
very low and so coupling only occurs in a small portion of the sample.
IN contrast, in a
13C NMR spectrum,
every 13C nucleus will be in molecules containing
1H nuclei so coupling does occur. However,
13C NMR spectrometers are usually set up to remove the coupling. As a result:
no coupling is seen in the 13C NMR spectra.