| Intermolecular
Forces Your feedback on these self-help problems is appreciated. Click here to send an e-mail. |
Shortcut to Questions |
|
|
Give a reason for the fact that, of the elements of Group 17, under standard conditions fluorine and chlorine are gases, while bromine is a liquid and iodine is a solid. |
||
| 2 |
Give a reason for the fact that water, the hydride of oxygen, is a liquid at room temperature while the hydride of sulfur (the next member of Group 16) is a gas? |
|
| 3 |
(1.) Hydrogen fluoride is a liquid at room temperature and a weak acid but hydrogen chloride is a gas and a strong acid. Explain these facts. (2.) For similar reasons water and ammonia have unexpected properties. What are they? |
|
| 4 |
Explain, using diagrams and a brief statement, how hydrogen bonding produces each of the following results: (1.) Ammonia gas is readily liquefied despite its low density. (2.) Acetic (ethanoic) acid is strongly associated in the liquid state. (3.) The boiling point of ethanol is much higher than that of dimethyl ether of identical molecular weight. |
|
| 5 |
List an example of each of the following types of species: (1.) a molecule containing no polar bonds. (2.) a non-polar molecule containing polar bonds. (3.) a polar molecule. (4.) a molecule subject to hydrogen bonding. (5.)
a compound containing the H- ion. |
|
| 6 |
Explain the following observations:
|
|
| 7 |
List the following substances in order of increasing normal boiling
point: |
|
| 8 |
List by formula the following substances in order of increasing boiling point: methoxymethane (dimethyl ether), CH3-O-CH3 |
|
| Intermolecular Forces - Answers | ||
| All group 17 elements (the halogens) have the same valence electron configuration and exhibit the same type of bonding. They all exist as covalent, diatomic molecules (F2, Cl2, Br2, I2). Between individual molecules there exists dispersion forces, which arise from the randomness of electron distribution within the individual molecules. Dispersion forces are intermolecular forces which are relatively weak when compared with covalent or ionic bonds, so the melting points of the halogens are low. The increase in melting point down the group is due to the increase in intermolecular dispersion forces experienced as a result of the increased number of electrons. The number of electrons in F2 is 18, Cl2 has 34, Br2 has 70, and I2 has 106. | ||
| 2 | The small size of the hydrogen atom and high electronegativity of oxygen result in highly polar O-H bonds in H2O. These highly polar bonds lead to extensive hydrogen bonding between water molecules. Sulfur is larger and less electronegative than oxygen, so the S-H bonds in H2S are much less polar and no hydrogen bonding between molecules occurs. These stronger intermolecular forces present between H2O molecules requires the supply of considerably more energy to break individual molecules from each other than is the case for H2S molecules - sufficient to give water a boiling point of 100 °C, while the weaker intermolecular forces present between H2S molecules results in a boiling point of only -60.3 °C (at 1 atm pressure). | |
| 3 |
(1.) Hydrogen fluoride exhibits hydrogen bonding between HF molecules. This results in a boiling point much higher than might be expected from consideration of molecular mass alone and thus hydrogen fluoride is a liquid at room temperature and pressure whereas the other hydrogen halides are all gases at those conditions. Ionisation in water is incomplete (unlike the other hydrogen halides), ie the dissociation equation
HF Strong hydrogen bonding between HF molecules and also between HF and H2O molecules leads to extensive association of HF molecules in water solution, and results in relatively few free H+ ions. Therefore HF is a weak acid. Conversely, HCl molecules do not hydrogen bond to each other or to water molecules, so it exists as a gas at room temperature and ionises completely in water solution (thus acting as a strong acid).
|
|
| 4 | 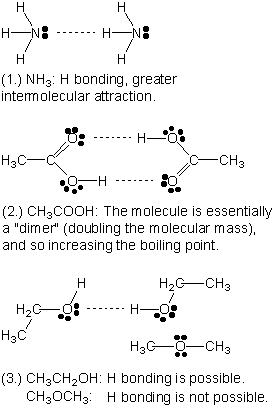 |
|
| 5 |
(1.) Molecules containing no polar bonds include H2, Br2, O3.
|
|
| 6 |
(1.) CCl4 would be expected to have a higher boiling point than CH4 since it posesses more electrons than CH4. Thus the magnitude of the dispersion forces present between CCl4 molecules is higher than that between CH4 molecules, and this is the main reason for the higher boiling point. Note that the increased molecular mass of CCl4 contributes only very slightly to the boiling point. The dominant factor is the increased dispersion force.
|
|
| 7 | In order
of increasing boiling point: HCl, HBr, HI, HF. The trend is determined by strength of dispersion force which is related to the number of electrons, except for HF, which exhibits hydrogen bonding sufficiently strong to more than compensate for the smaller number of electrons in the HF molecule. This results in a boiling point higher than even the most electron rich hydrogen halide, HI. |
|
| 8 |
The C-O bonds of methoxymethane (dimethyl ether) (CH3-O-CH3) are polar. The geometry of the molecule is angular, resulting in an overall molecular dipole. Hence the molecule will be subject to dipole-dipole and dipole/induced dipole interactions as well as the stronger dispersion forces.
|
|