|
Electrolysis Your feedback on these self-help problems is appreciated. Click here to send an e-mail. |
Shortcut to Questions |
|
|
How many (a) faradays,
(b) coulombs are required to deposit 0.587 g of Ni from a
solution of Ni2+ ? |
||
| 2 |
From a dilute solution of potassium tricyanocuprate(I),
K2[Cu(CN)3], a uniform current deposits 0.586 g of copper in 108 minutes. What
volume of hydrogen measured over water at 296 K and 746.0 mmHg would
the same current liberate from dilute sulfuric acid in 81.0 minutes? |
|
| 3 |
In the electrolysis of 0.100 M copper(II) sulfate solution, copper is deposited. How long would it be necessary to pass a 0.100 ampere current in order to deposit all of the copper from 1.00 litre of this solution? |
|
| 4 |
Three electrolysis cells are connected in series. They contain, respectively, solutions of copper(II) nitrate, silver nitrate, and chromium(III) sulfate. If 1.00 g of copper is electrochemically deposited in the first cell, calculate the mass of silver and chromium deposited in the other cells. |
|
| 5 |
A constant current of 3.7 milliampere is passed through molten sodium chloride for 9.0 minutes. The sodium produced is allowed to react with water (500 mL). What is the pH of the resulting solution? |
|
| 6 |
A constant current of 0.126 ampere was passed through a dilute sulfuric acid solution for 149 minutes. The hydrogen produced was collected over mercury and the pressure adjusted to that of the surroundings (751 mmHg). The room temperature was 291 K. What was the volume of hydrogen produced? |
|
| 7 |
One of the experimental ways of determining the Avogadro constant is by electrolysis. A current of 1.00 ampere is passed for 1.00 hour through a solution of potassium iodide. The weight of iodine liberated at the anode is 4.7135 g and there is no other anodic product. If the charge on the electron is 1.602 x 10-19 coulomb, find from this set of data the Avogadro constant, NA, correct to three significant figures. The atomic weight of iodine is 126.90. |
|
| 8 |
Two electrolytic cells each containing two platinum electrodes were connected in series and a current of 0.500 ampere was passed for 16 minutes and 5 seconds. Cell 1 contained 0.100 M AgNO3 and cell 2 contained 0.100 M CuSO4. Calculate the following: (a)
The change in mass of the platinum cathode in cell 1. |
|
| Electrolysis (Answers) | ||
|
The reaction equation
is One coulomb of charge
(C) is the total charge involved when a current of one amp flows
for one second. i.e. 1 amp = 1 coulomb flowing for 1 second. |
||
| 2 | The
first task in this question is to calculate from the amount of copper
released, the current used in the electrolysis reactions. Then in
the second part, knowing the current and the time it flows, the moles
of electrons supplied to the H+ ions can be deduced. Then
the moles of H2 released and its volume under the stated
conditions can be obtained. From the first electrolysis, deduce the moles of electrons: The reaction equation is Cu+ + e- → Cu from which, one mole of electrons releases one mole of Cu. Mass of copper = 0.586 g ∴ moles of Cu = 0.586 / 63.55 = 9.221 x 10-3 mol and moles of electrons also = 9.221 x 10-3 mol. Convert moles of electrons to coulombs: Q = F x moles of electrons = 96487 x 9.221 x 10-3 C = 8.897 x 102 C From the coulombs of charge, calculate the current: By the definition of the coulomb (see Q 1 answer), charge in coulombs = current in amps x time in seconds or Q = I x t ∴ I = Q / t = 8.897 x 102 / (108 x 60.0) =0.1373 amps Note that time must be expressed in seconds as required by the definition of the coulomb. Calculate the moles of electrons this current provides in 81.0 minutes: From Q = I x t, in 81.0 minutes (= 81.0 x 60.0 s), Q = 0.1373 x 81.0 x 60.0 = 6.673 x 102 C. Converting coulombs to moles of electrons, moles of electrons = coulombs / F = 6.673 x 102 / 96487 = 6.916 x 10-3 mol. Calculate the moles of H2 released: The reaction equation is 2H+ + 2e- → H2 from which, one mole of H2 formed requires two moles of electrons. ∴ moles of H2 = ½ x 6.916 x 10-3 = 3.458 x 10-3 mol Calculate the volume of hydrogen gas formed: From the ideal gas equation PV = nRT, ∴ V = nRT / P n = 3.458 x 10-3 mol R = 8.314 J K-1 mol-1 T = 296 K P = (746.0 - 20.9) mmHg = 725.1 mmHg or (725.1 / 760.0) atm or (725.1 / 760.0) x 101.3 kPa = 96.65 kPa. (Note that the total pressure must be reduced by the equilibrium vapour pressure of the water over which the hydrogen is collected in order to obtain the true pressure of hydrogen in the container.) Substituting this data, V = (3.458 x 10-3 x 8.314 x 296) / 96.65 = 8.80 x 10-2 L |
|
| 3 |
The reaction equation
is |
|
| 4 |
The reaction equations
are From the stoichiometry of these equations, one mole of electrons would deposit ½ mole of Cu in the electrolysis. As the cells are in series, the same current flows through all three cells and one mole of electrons would produce one mole of Ag and 1/3 mole of Cr from their solutions respectively. The moles of electrons
used = 2 x moles of Cu deposited. so moles of electrons
passed = 2 x 1.574 x 10-2 = 3.148 x 10-2 mol. In the
silver cell:
|
|
| 5 |
The reaction equation
is |
|
| 6 |
The reaction equation is |
|
| 7 |
The reaction equation
is = 1.8572 x 10-2
mol = 2.247 x 1022
electrons |
|
| 8 |
Terminology: 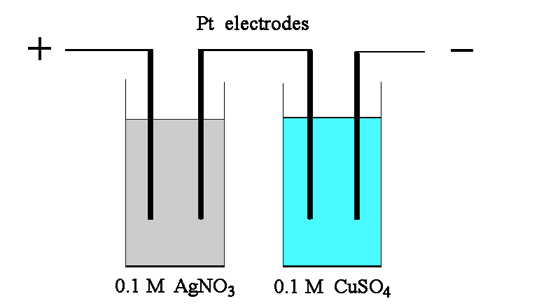
= 2.50 x 10-3
mol. |
|