The electromagnetic spectrum shows the range of energies (or equivalently frequency or wavelength) of electromagnetic waves. Familiar types of electromagnetic waves include (in increasing energy) radio waves, microwaves, infrared, visible light, ultra violet, X-rays and gamma rays.
Like all waves, the type of electromagnetic radiation is characterized by its:
- Frequency n (measured in Hertz (Hz)): the number of waves passing each second,
- Wavelength l (measured in metres or, more conveniently, nanometres (1 nm = 10-9 m)): the distance from peak to peak (or from trough to trough),
- Speed (measured in ms-1) : the distance travelled by the wave every second and
- Amplitude: the height of the peak (or trough).
All electromagnetic waves travel at the speed of light, c = 2.99 x 108 ms-1.
The speed, wavelength and frequency of light are linked:
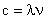 | (1) |
The energy of an electromagnetic wave is related to its frequency by Planck's equation:
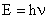 | (2) |
where h = 6.634 x 10-34 Js. The energy of electromagnetic radiation depends on its frequency. The
intensity or brightness depends on its amplitude.
The table below shows the approximate wavelengths, frequencies and energies for selected regions of the electromagnetic spectrum.
Region |
Wavelength (nm) |
Frequency (Hz) |
energy (kJ mol-1) |
| |
|
|
|
Radio |
> 108 |
< 3 x 109 |
< 0.01 |
Microwave |
108 - 105 |
3 x 109 - 3 x 1012 |
0.01 – 1 |
Infrared |
105 - 700 |
3 x 1012 - 4.3 x 1014 |
1 -170 |
Visible |
700 - 400 |
4.3 x 1014 - 7.5 x 1014 |
170 - 300 |
Ultraviolet |
400 - 1 |
7.5 x 1014 - 3 x 1017 |
300 – 105 |
X-Rays |
1 - 0.01 |
3 x 1017 - 3 x 1019 |
105– 107 |
Gamma Rays |
< 0.01 |
> 3 x 1019 |
> 107 |
|
In chemical terms, the different parts of the spectrum have different affects on atoms and molecules. Radio and microwave radiation causes molecules to rotate. Infrared radiation causes bonds to stretch and bend leading to us feeling heat. X-rays and gamma rays cause electrons to be completely removed and can lead to molecules breaking up or rearranging, sometimes with harmful consequences to the body.
The most familair part of the spectrum is visible light. Visible and ultraviolet light causes electrons to become excited, giving rise to colours and promoting bonds to break and reactions to occur. More information on the relationship between colour and light is available.
