|
Introduction to Structure Determination | ||||||||||||||||||||||||||||
| Ultraviolet: Introduction | |||||||||||||||||||||||||||||
|
|
Radiation in the ultraviolet region of the electromagnetic spectrum has the energy to excite electrons from one energy level to another. Core electrons and those in σ bonds are bound too tightly to be excited by radiation in the ultraviolet region. Excitation of these electrons can only be achieved using higher energy radiation, such as X-rays. The electrons in π bonds are less tightly held and if an organic molecule possesses conjugated π bonds, ultraviolet and even visible light will be able to excite the electrons in these bonds.
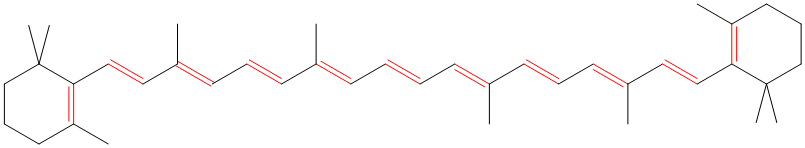
ConjugationConjugation occurs when a molecule contains alternating double (or triple) and single bonds. The double bonds can be C=C, C=O or C=N and the triple bonds can be C≡C or C≡N. These multiple bonds must be separated by only one single bond. If the multiple bonds are separated by more than one single bond, no conjugation is possible.An alternative (and equivalent) definition is that conjugation occurs when there is a sequence of four or more sp2 or sp hybridised atoms in a row. The more bonds they are in the sequence of alternating double (or triple) and single bonds, the greater the extent of the conjugation. As the conjugation increases, the energy required to excite the electrons decreases. Many natural and synthetic dyes and pigments contain long conjugated systems as the energy required to excite the π electrons is low enough to fall in the visible part of the spectrum. Β-carotene, for example, contains a sequence of 11 alternating double and single bonds and is responsible for the orange colour of carrots. The table below shows how the extent of the conjugation affects the wavelength of the radiation needed to excite an electron. Where conjugation is present, the bonds involved in the conjugation are shown in red.
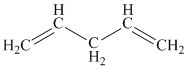
Note the effect of conjugation: both penta-1,4-diene and penta-1,3-diene contain two C=C bonds but only the latter is conjugated. This increases the wavelength of the light required to excite a π electron from 177 nm to 225 nm. |
||||||||||||||||||||||||||||
| © Prof Adam Bridgeman, School of Chemistry, The University of Sydney, 2024 |
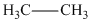
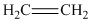
-penta-1,3-diene.gif)
-hexa-1,3,5-triene.gif)