|
Introduction to Structure Determination | ||||||||||||||||||||||||||||
| NMR: Introduction | |||||||||||||||||||||||||||||
|
|
NMR Spectrometer
The protons and neutrons that make up the nucleus are arranged in energy levels, just like the electrons that orbit around it. Like electrons, protons and neutrons possess spin. The spins of the protons and neutrons can be paired or unpaired so that some nuclei. If all are paired, the spins cancel and the nucleus does not have an overall spin. In a nucleus with an odd number of protons and neutrons, such as 1H (1 proton and 0 neutrons) or 13C (6 protons and 7 neutrons), it is not possible for all the spins to be paired and the nucleus is left with unpaired spins and has an overall spin. A nucleus with a spin behaves like a spinning charge. A spinning charge generates a magnetic field: nuclei with spin act like small bar magnets. NMR spectrometers contain very strong magnets. Inside the magnetic field this generates, the spin of the nucleus inside a 1H or 13C atom can either be aligned with the field (low energy) or against the magnetic field. The picture below shows what happens to the energy of these two arrangements as the magnetic field incrases. The energy difference between the two arrangements increases as the strength of the magnetic field increases. In the Earth's magnetic field, approximately 50 µT, the energy difference is very, very small. NMR spectrometers use magnetic fields which are much larger, between 1 and 20 T. Even so, the energy separation is small and radiofrequency radiation is enough to excite the nucleus and change its alignment with the field. In NMR spectroscopy, the energy difference between the two arrangements of the nuclear spin is measured. Radiofrequency radiation with energy which exactly matches the energy separation is absorbed by the nucleus ("resonance"). 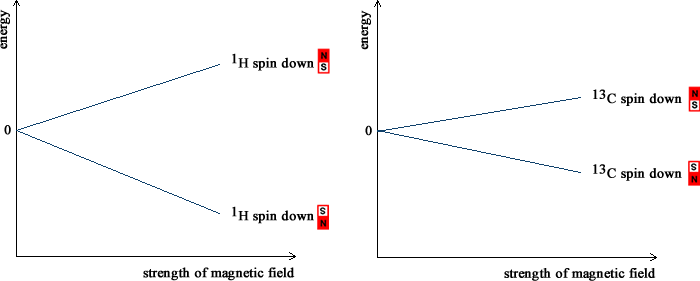
As illustrated in the picture above, the energy separation between the two arrangements is greater for a 1H nucleus than for a 13C nucleus if the same magnetic field is applied. Higher energy radiofrequency radiation is needed to excite the 1H nucleus than the 13C nucleus. The NMR spectrum due the 1H nuclei in a molecule occurs at quite different frequencies than that due to the 13C nuclei. This means that it is possible to record the two spectra separately. The most commonly recorded NMR spectra are those of 1H and 13C. The low abundance of 13C presents some technological difficulties but these have been overcome because of its importance. Because of their presence in some biochemically important molecules, 19F and 31P NMR spectrs are sometimes recorded. Other nuclei are also sometimes recorded for more specialized applications. As detailed on the 'chemical shifts' page, the electrons around a nucleus shield some of the magnetic field. The magnetic field felt at each nucleus in a molecule depends on the distribution of the electrons in the molecule. The exact energy of the radiofrequency radiation may be slightly different for each nucleus and depends on the bonds and electrons that surround. NMR is able to give information on the environment around each nucleus. As well as the magnetic field from the NMR spectrometers, the nuclei with spin also experience each others' magnetism. As detailed on the 'coupling' page, these interactions give direct information on the surrounding nuclei and hence on which atoms are connected to each other. Whilst the energy of the radiofrequency radiation that is absorbed gives information on the chemical environments in a molecule and the atom-to-atom connectivity, the amount of radiation absorbed is depends on the number of nuclei. The size of the peaks in the 'spectrum' can be used to measure the number of nuclei of each type. When put together, this information leads to a very detailed picture of the molecular geometry and NMR is invaluable in chemistry and biochemistry. |
||
| © Prof Adam Bridgeman, School of Chemistry, The University of Sydney, 2024 |