Spectroscopy is concerned with the measurement of the energy difference between the energy levels of a molecule. A molecule can increase its rotational, vibrational or electronic energy by absorbing light or emitting light.
Microwaves excite molecular rotations, infrared light excites molecular vibrations and visible/ultraviolet light excites electrons. Microwaves, infrared, visible and ultraviolet light all form part of the
electromagnetic spectrum:
-
they act like waves and all travel at the speed of
light, c = 2.99 x 108 m s-1,
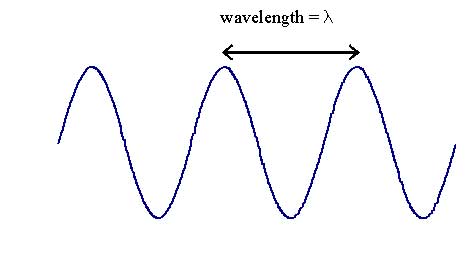
- the number of waves arriving each second is the frequency, ν (Greek letter 'nu') measured in Hertz (s-1)
- they have wavelength λ (Greek letter 'landa'), measured in metres or, more conveniently nanometres (1 nm = 1 x 109 m)
The speed, wavelength and frequency of light are linked:
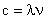 | (1) |
or |
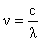 | (2) |
The light absorbed or emitted by a molecule is often reported either in Hertz (frequency) or in nanometres (wavelength).
Planck's equation relates the frequency of light to its energy (in Joules):
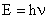 | (3) |
where h = 6.634 x10-34 Js.
The frequency is proportional to the energy difference between the energy levels of the molecule. The energy difference is just the frequency multiplied by Planck's constant h. For this reason, spectroscopists often refer to the frequency as a unit of energy.
Combining equations (3) and (2) gives the energy in terms of the wavelength:
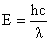 | (4) |
The wavelength is inversely proportional to the
energy difference. Alternatively, the energy difference is proportional to
1/λ. An alternative unit to the frequency is the
'wavenumber', 1/λ.
It is usually more convenient to use cm-1 instead of m-1.
Converting between different spectroscopic units
The calculator below converts between the common (and some of the less commonly) used units in spectroscopy. Enter a value to be converted and choose its units from the list. Press 'convert' to convert the number into the available units.
To enter a large or small number using scientific notation - for example, enter 1.9e2-27 for 1.9x10-27 and 6.03e23 for 6.03x1023.
