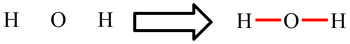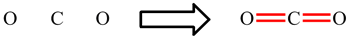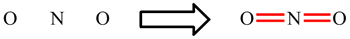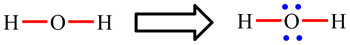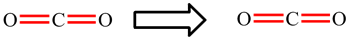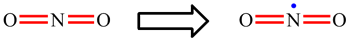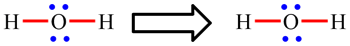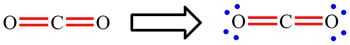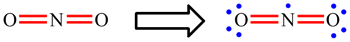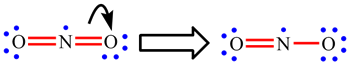Drawing Lewis Structures |
 Video tutorial |
 | Introduction to Lewis structures: |
| Lewis structures show how the electrons are arranged in the molecule. The diagram opposite shows the Lewis structure for the water molecule, H2O, and the ethene molecule, C2H4. Lines represent bonds: single lines are single bonds (such as the O-H and C-H bonds) and two lines are double bonds (such as the C=C bond). Dots represent electrons which are not involved in bonding. Most commonly, two occur in pairs and then represent non-bonding or lone pairs (such as the lone pair on O in H2O). |
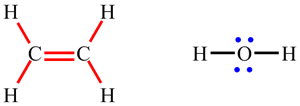
|
For some molecules, there is more than one way of describing the arrangement of the electrons. When this is possible, there are multiple Lewis structures called resonance structures and the 'real' arrangement is an average of all the possible resonance structures. We will use a step by step approach which can be used to work out the Lewis structure of simple molecules. By working through the examples, you'll get the hang of it and then will be able to tackle larger molecules, including organic ones.
 | Work out the number of bonds made by the central atom: |
The 'central atom' is the one that we are interested in drawing the bonds to. In a simple molecule like H2O, it is the one at the centre of the molecule (oxygen in H2O). In a larger molecule such as ethene C2H4, we might look at the bonding around each central atom (for example each carbon) in turn. This tutorial covers only simple XYn type molecules.
If you've forgotten how to work out which is the central atom in a molecule from its formula, work back through the iChem tutorial on the rules for writing formulas of compounds.
In most molecules, like CH4, BF3 and SF6, the central atom is the first one in the formula. In molecules with an electronegative element bonded to hydrogen, like H2O and H2S, it is the element which isn't hydrogen.
The number of bonds the central atom makes is its valency. In a XYn molecule, the valency of the central atom X is equal to the absolute value of its oxidation number. For example, in H2O, oxygen has an oxidation number of -2 so its valency is 2. In SF6, the oxidation number of sulfur is +6 so its valency is 6.
Working out the oxidation number is straightforward - use the iChem tutorial on oxidation numbers to help with this.
Once you know the valency, you know the number of bonds that the central atom will make.
If you've forgotten how to work out which is the central atom in a molecule from its formula, work back through the iChem tutorial on the rules for writing formulas of compounds.
In most molecules, like CH4, BF3 and SF6, the central atom is the first one in the formula. In molecules with an electronegative element bonded to hydrogen, like H2O and H2S, it is the element which isn't hydrogen.
The number of bonds the central atom makes is its valency. In a XYn molecule, the valency of the central atom X is equal to the absolute value of its oxidation number. For example, in H2O, oxygen has an oxidation number of -2 so its valency is 2. In SF6, the oxidation number of sulfur is +6 so its valency is 6.
Working out the oxidation number is straightforward - use the iChem tutorial on oxidation numbers to help with this.
Once you know the valency, you know the number of bonds that the central atom will make.
Examples:
|
| |||||||||
|
| |||||||||
 | Work out the number of lone electrons on the central atom: |
The number of unused electrons on the central atom can easily be calculated:
number of electrons left over = number of valence electrons - valencyTo get the number of valence electrons, count over from left to right on the Periodic Table to the element (ignoring the transitions metals). Alternatively, for the s and d-block elements, it is simply the group number and for the p-block elements it is the group number minus 10. These electrons occur in pairs (lone pairs) if possible. If there is an odd number of electrons, there must be an unpaired electron.
Examples:
|
| |||||||||
|
| |||||||||
 | Add electrons to the outer atoms: |
The 'outer atoms' are those which are attached to the central atom.
The commonest outer atoms you will meet are hydrogen and the electronegative elements (N, O, S, F, Cl, Br and I). You can assume that these atoms will have complete shells. These come from the bond(s) they make to the central atom plus lone pairs.
Each bond = 2 electrons. A single bond = 2 electrons, A double bond = 4 electrons. A triple bond = 6 electrons.
Each bond = 2 electrons. A single bond = 2 electrons, A double bond = 4 electrons. A triple bond = 6 electrons.
- H can only have 2 electrons in its shell. It will already be making 1 bond to the central atom so already has a full shell.
- N, O, S, F, Cl, Br and I will have 8 electrons around them.
- They may be making single, double or even triple bonds to the central atom giving them 2, 4 or 6 electrons respectively.
- To get up to 8 electrons, they must therefore have 6, 4 or 2 electrons respectively.
- These are in lone pairs so correspond to 3, 2 or 1 lone pairs.
Examples:
|
| |||||||||
|
| |||||||||
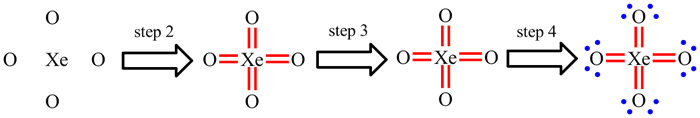
 | If central atom is C, N or O: |
The elements of the second row (Li - Ne) can only have a maximum of 8 electrons - the octet rule. You will commonly meet molecules with C, N or O as the central atom. The process described above may lead to more than 8 electrons on one of these atoms. You should quickly check that the octet rule is being obeyed.
For atoms in the third and lower rows, the octet rule is irrelevant and this step does not need to be applied.
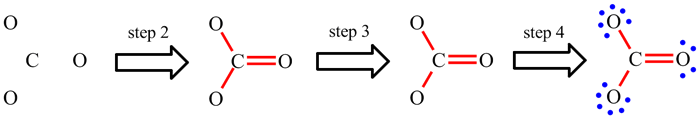
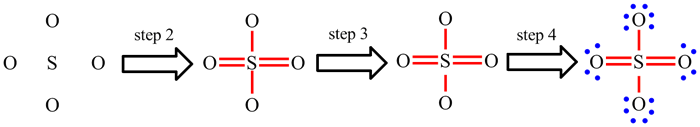
The only way to get to grips with Lewis structures and these steps is to work through lots of examples and to practice, practice, practice.
For atoms in the third and lower rows, the octet rule is irrelevant and this step does not need to be applied.
Examples:
In H2O, the oxygen atom is making 2 single O-H bonds and has 2 lone pairs: 2 × 2 electrons (single bonds) + 2 × 2 electrons (lone pairs) = 9. The octet rule is obeyed.
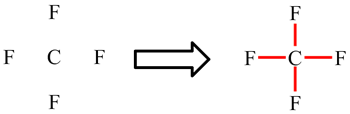
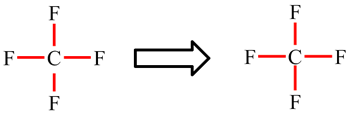
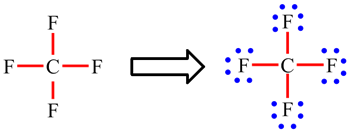
In CF4, the carbon atom is making 4 single bonds and has no lone pairs: 4 × 2 electrons (single bonds) = 8. The octet rule is obeyed.
In CO2, the carbon atom is making 2 double bonds and has no lone pairs: 2 × 4 electrons (double bonds) = 8. The octet rule is obeyed.
In H2O, the oxygen atom is making 2 single O-H bonds and has 2 lone pairs: 2 × 2 electrons (single bonds) + 2 × 2 electrons (lone pairs) = 9. The octet rule is obeyed.
In CF4, the carbon atom is making 4 single bonds and has no lone pairs: 4 × 2 electrons (single bonds) = 8. The octet rule is obeyed.
In CO2, the carbon atom is making 2 double bonds and has no lone pairs: 2 × 4 electrons (double bonds) = 8. The octet rule is obeyed.
|
The only way to get to grips with Lewis structures and these steps is to work through lots of examples and to practice, practice, practice.
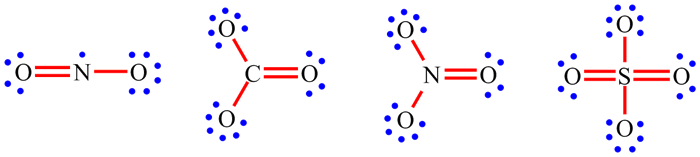
 | Examples: |
|
||||
|
||||
|
||||
|
||||
 | Resonance: |
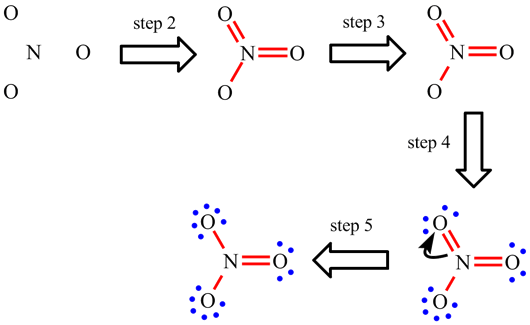
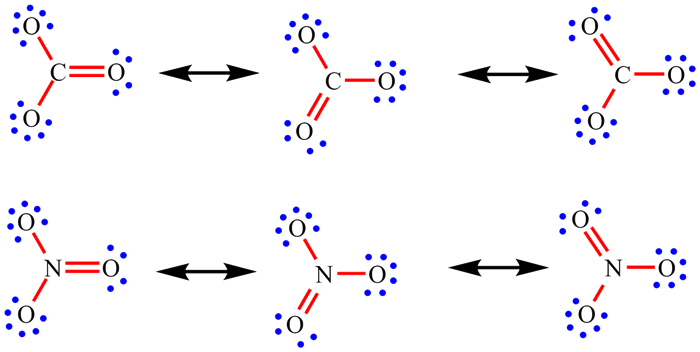
| When working through the examples on NO2, CO32-, NO3- and SO42-, you may have now noticed that the placement of the single and double bonds seemed completely arbitrary. |
 |
| Rather than being a weakness of the approach, when this happens it is actually telling us something. If there is more than one Lewis structure which can be drawn, all are important. Each separate one is called a resonance structure. For CO32- and NO3-, for example, there are three equivalent resonance structures. These are drawn below with the double headed arrow used to show that they are resonance structures. The term "resonance" is historic and a little misleading. The electrons do not really hop between the difference positions and the bonds are not changing between being single and double. The real arrangement is an average of all the possible resonance structures. For these two ions, this means that each bond is actually the same and each will have a strength and length which intermediate between a single and double bond. |
 |
 | Self learning quizzes: |
These quizzes enable you to graphically build Lewis structures and to test your understanding:
Lewis structure maker