Converting between concentration, moles and volume
Many chemical reactions, including the vast majority of those that happen in the human body, occur in solution.
Concentration is used to describe the composition of a solution. The concentration is the amount of stuff that is present. A highly concentrated solution contains a great deal of stuff and a very dilute solution contains very little! Their properties will be very different.
The concentration is defined as the amount of
solute dissolved in a particular volume of the
solvent.
molarity. Concentration is indicated using square brackets around the substance to which it refers to. It has units of mol L
-1 or M. (Try not to confuse the molarity unit "M" with the symbol for molar mass "
M" - italics are used to symbols and are not used to units.) When performing any calculations in science, an appreciation of the accuracy and significance of the measurements is vital - just ask an athlete under suspicion of taking performance enhancing drugs. If you are unfamilar with the proper way to handle signficant figures, read the
iChem online tutorial (identical to the E9 week 4 pre-laboratory information)
Dealing with concentrations is very important and you will do so
many times. There are three possible scenarios:
| concentration (in mol L-1) = | amount (in mol) | or | c = | n |
| volume (in L) | V |
| amount (in mol) = concentration (in mol L-1) × volume (in L) | or | n = c × V |
| volume (in L) = |
amount (in mol) | or |
V = |
n |
| concentration (in mol L-1) |
c |
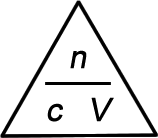 |
Use a finger to cover up the quantity you want to calculate. The other two values show you how to do the calculation.
- To calculate the concentration, cover up c and you are left with n / V. Hence, c = n / V
- To calculate the number of moles, cover up n and you are left with c V. Hence, n = c × V
- To calculate the volume, cover up V and you are left with n / c. Hence, V = n / c
|
4. A 550 mL solution of calcium hydroxide containing 0.25 moles of Ca(OH)2 has [OH-] = 0.91 M:
As 1000 mL = 1 L, 550 mL = 0.55 L. Ca(OH)
2 dissolves to give
one Ca
2+ and
two OH
-(aq). Hence 0.25 mol of Ca(OH)
2 will dissolve to give 0.25 mol of Ca
2+ and 0.50 mol of OH
-. Hence,
| Using c = | n |
, [OH-] = | 0.50 mol | = 0.91 mol L-1 = 0.91 M |
| V | 0.55 L |
Note that the measurements (550 mL and 0.25 mol) are both known to 2 significant figures. The accuracy of the final answer is limited by the
least accurately known measurement and so the answer has been given to 2 significant figures.
7. A 250 mL solution with [H+] = 0.10 M requires 0.13 mol of sulfuric acid
As 1000 mL = 1 L, 250 mL = 0.25 L. With [H
+] = 0.1 M = 0.1 mol L
-1,
| Using n = c × V, amount of H+ = (0.1 mol L-1) × (0.25 L) = 0.025 mol |
One H
2SO
4 ionizes to give
two H
+(aq) ions. Hence 0.025 mol of H
+ requires 0.0125 mol of H
2SO
4.
The measurements (250 mL and 0.10 M) are both known to 2 significant figures. The accuracy of the final answer is limited by the
least accurately known measurement. Hence to 2 significant figures, 0.013 mol of sulfuric acid is required.
8. A 12 mL solution with [OH-] requires 0.01 mol of calcium hydroxide:
As 1000 mL = 1 L, 12 mL = 0.012 L. With [OH
-] = 0.2 M = 0.2 mol L
-1,
| Using n = c × V, amount of OH- = (0.2 mol L-1) × (0.012 L) = 0.024 mol |
Ca(OH)
2 dissolves to give
two OH
-(aq) ions. Hence 0.024 mol of OH
- requires 0.012 mol of Ca(OH)
2.
The measurements, 12 mL and 0.2 M, are known to 2 and 1 significant figures respectively. The accuracy of the final answer is limited by the
least accurately known measurement. Hence to 1 significant figure, 0.01 mol of calcium hydroxide is required.
11. 0.75 L of a 2.0 M H+ solution contains 1.5 mol of H2SO4:
As H
2SO
4 ionizes to give
two H
+, 1.5 mol of H
2SO
4 will produce 3.0 mol of H
+. As the solution has [H
+] = 2.0 M = 2.0 mol L
-1, the volume that contains 3.0 mol of H
+ can be found
| Using V = | n |
, volume of solution = | 3.0 mol | = 1.5 L |
| c | 2.0 mol L-1 |
12. 4.0 L of a 0.20 M OH- solution contains 0.40 mol of Ca(OH)2:
As Ca(OH)
2 ionizes to give
two OH
-, 0.40 mol of Ca(OH)
2 will produce 0.80 mol of OH
-. As the solution has [OH
-] = 0.20 M = 0.20 mol L
-1, the volume that contains 0.80 mol of OH
- can be found
| Using V = | n |
, volume of solution = | 0.80 mol | = 4.0 L |
| c | 0.20 mol L-1 |
The measurements (0.20 M and 0.40 mol) are both known to 2 significant figures. The accuracy of the final answer is limited by the least accurately known measurement. Hence to 2 significant figures, the volume is 4.0 L.


