Acid/Base Titrations
Here is an example of an experiment in which a KOH solution of unknown concentration, but approximately 0.1M, is titrated against a standard HNO3 solution which is 1.036M.
Experiment
- The standard solution is diluted 10 fold by taking 25.00 mL of the nitric acid in a pipette which has previously been rinsed with 1.036 M nitric acid. The 25.00 mL is transferred to a volumetric flask, previously rinsed with deionised water, and the volume made up to exactly 250.0 mL with deionised water. The concentration of this solution is 0.1036 M.
- The diluted nitric acid is introduced into a burette, previously rinsed with 0.1036 M nitric acid and the level adjusted to 0.00 mL
- 25.00 mL of the potassium hydroxide solution is transferred by pipette to a conical flask, previously rinsed with deionised water and two drops of methyl-orange indicator added.
- The solution is rapidly titrated until the indicator changes from yellow to pink in colour. The end point is reached after the addition of approximately 24 mL of acid.
- A series of accurate titrations is performed until three reading within 0.05 mL are obtained. In each case about 20 mL of acid is added rapidly from the burette and the remainder added slowly in progressively decreasing volumes down to single drops. As the end point is neared, shown by a slight change in colour of the indicator, part-drops are detached from the tip of the burette by touching it against the neck of the conical flask and washing down the inside of the flask with deionised water.
- A series of "titers" is obtained: 22.75, 22.65, 22.70 mL giving a mean reading of 22.70 mL known concentration.
 |
Calculations
- The equation for the reaction is
HNO3 + KOH 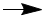
KNO3 + H2O - Moles of nitric acid used is
(concentration/M) x (volume/L) = (0.1036 mol L-1 )(22.70 x 10-3 L) = 2.35172 x 10-3 mol. (All figures have been retained).
- As 1 mole of nitric acid reacts with 1 mole of potassium hydroxide, amount of potassium hydroxide = 2.35172 x 10-3 mol.
- This is from 25.00 mL of solution, so
[OH-] = (2.35172 x 10-3 mol / (25.00 x 10-3 L) = 0.940688 M = 0.9407 (4 s.f. to reflect accuracy of measurements).