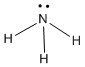
A complex normally consists of a monatomic positive ion ( ie a metal ion) surrounded by a number of ligands . Each ligand is a molecule or ion, generally containing at least one atom with an unshared pair of electrons. Such an electron pair permits the formation of a metal-ligand bond with partial covalent character. The atom of the ligand that is involved in this interaction is the donor atom . The function of the unshared pair is to allow for the partial covalent character of the metal-ligand bond. Common ligands include the hydroxide ion (OH–), water molecule (H2O), ammonia molecule (NH3), cyanide ion (CN–) and halide ions (F–, Cl–, Br– and I–).
|
|
|
|
|
hydroxide |
water |
ammonia |
cyanide |
chloride |
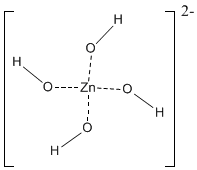
Most complexes carry a positive or negative charge, and are called complex ions . An example of a complex-ion is the following, in which because the ligands are negatively charged, the charge of the complex-ion is negative.
|
|
One unshared pair from the O atom of each OH– ion is directed towards the Zn2+ ion, the O atoms being arranged tetrahedrally about Zn2+. Due to repulsion of the electron pairs of the donor-atoms, the arrangement of ligands in a complex ion is symmetric.
The following arrangements of donor-atoms commonly occur.
Number of donor atoms |
Arrangement of donor atoms around the metal ion: |
|
|
2 |
linear |
4 |
tetrahedral |
6 |
octahedral |
The number of donor-atoms bound to the metal ion is called the co-ordination number or CN.
Examples
Name of Complex |
Formula |
Metal Ion |
Ligand |
CN |
Geometry |
|
|
|
|
|
|
tetrahydroxozincate(II) ion |
[Zn(OH)4]2– |
Zn2+ |
OH– |
4 |
tetrahedral |
hexaamminecobalt(III) ion |
[Co(NH3)6]3+ |
Co3+ |
NH3 |
6 |
octahedral |
hexaaquanickel(II) ion |
[Ni(H2O)6]2+ |
Ni2+ |
H2O |
6 |
octahedral |
tetrachloromercurate(II) ion |
[HgCl4]2– |
Hg2+ |
Cl– |
4 |
tetrahedral |
hexacyanoferrate(II) ion |
[Fe(CN)6]4– |
Fe2+ |
CN– |
6 |
octahedral |
hexacyanoferrate(III) ion |
[Fe(CN)6]3– |
Fe3+ |
CN– |
6 |
octahedral |
triamminetrichlorocobalt(III) ion |
[Co(NH3)3Cl3] |
Co3+ |
NH3, Cl– |
6 |
octahedral |
dicyanoargentate(I) ion |
[Ag(CN)2]– |
Ag+ |
CN– |
2 |
linear |
Many complexes of transition metal ions are coloured. For example, when a solution containing Cu2+ is added to NH 3 solution, an intense purple solution forms. This indicates the probable formation of a complex ion. Complex ions can be isolated as salts. In this case, the sulfate salt of the copper(II)/ammonia complex is soluble in water but insoluble in ethanol. It is therefore convenient to isolate the salt from water solution by the addition of ethanol.
The number of donor atoms that group themselves around a metal ion of charge 2+ is usually 4 or 6. Since NH3 molecules have no charge the NH3 complex of Cu2+ will have a charge of 2+. In this case, there are four NH3 groups coordinated to the Cu2+ ion. This ion tends to have a co-ordination number of 6, so there may also be two water molecules co-ordinated to the Cu2+ ion, thus the complex ion may be formulated as [Cu(NH3)4(H2O)2]+.
When the complex ion is isolated as the sulfate salt, some H2O molecules may also be contained in the crystal lattice as the complex sulfate salt is allowed to crystallise. Therefore, the isolated complex salt may be formulated as [Cu(NH3)4(H2O)2]SO4· xH2O, where x is the number of H2O molecules in the crystal lattice. .