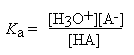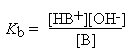General Acid/Base Theory
Acids
A typical weak acid, HA, reacts with water according to the following equation:
HA + H2O
H3O+ + A-
The equilibrium constant for this reaction is called the acid dissociation constant and is given by:
The stronger acids have the higher values of Ka. Indeed, for strong acids the reaction proceeds essentially 100% to the right and thus Ka is very large. The vast majority of organic acids, however, have K a values smaller than 10-3 M. In order to avoid dealing with these small numbers, it is common practice to tabulate acid strengths as pKa values, where:
pKa = -log10 Ka .
The weakest acids have the smallest Ka values. Hence, the weakest acids have the largest pKa values and the strongest acids have the smallest (or negative) pKa values.
Sodium hydroxide is a strong base and dissociates completely in water to produce OH- ions. These OH- ions react with a weak acid essentially to completion, as follows:
HA + OH-
H2O + A-
Bases
A typical weak base, B, reacts with water according to the following equation:
B + H2O
HB + OH -
The equilibrium constant for this reaction, called the base dissociation constant , is given by:
The stronger bases have the higher values of Kb. The vast majority of organic bases have Kb values smaller than 10-3 M, and it is common practice to tabulate base strengths as pKb values, or even more commonly to merely tabulate the pKa values of the conjugate acids, from which the Kb can be deduced:
pKb = -log10 Kb .
For a conjugate acid base pair at 298 K, HA and A-:
Ka (HA) x Kb (A-) = 10-14The strongest bases have the smallest pKb values and hence their conjugate acids have the largest pKa values.
and
pKa (HA) + pKb (A-) = 14
Hydrochloric acid is a strong acid and dissociates completely in water to produce H+ and Cl- ions. The H+ ions react, essentially to completion, with a base as follows:
B + H+
HB+