Preparation of Aspirin (Acetylsalicylic Acid) and Thin-Layer Chromatography of Analesic Drugs
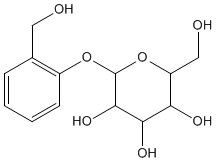
Salicin, a glycoside of salicylic acid present in the leaves and bark of willow trees, has been used for centuries in a variety of herbal remedies. In vivo, it is converted into salicylic acid which acts to reduce inflammation and lower the temperature of patients suffering from fever.
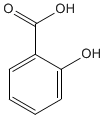
Salicylic acid itself is unsuitable as a drug since large doses have an unpleasant taste and also cause gastric irritation. These problems were largely overcome by the introduction of acetylsalicylic acid (aspirin) by the German company Bayer in 1899. Aspirin is an ester which passes through the stomach unchanged before being hydrolysed by the basic medium of the intestine to form the active compound.
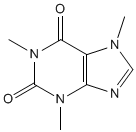
salicin |
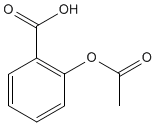
aspirin |
salicylic acid |
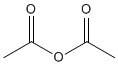
|||
|
|
|
|||
Esterification
When a carboxylic acid reacts with an alcohol (such as ethanol or a phenol), the products are water and an ester. The esterification reaction is slow and as soon as the products begin to form, the reverse reaction, hydrolysis, begins. An equilibrium is finally attained with all reactants and products present.
RCOOH + R'OH
RCOOR' + H2O
At 20 °C the rate of reaction in both the forward and the backward directions is slow and it takes many days to attain equilibrium. This is overcome by heating the mixture. Doing this increases the rate of both the forward and reverse reactions, thus achieving the equilibrium state much faster. In addition, the value of K at room temperature is typically only about 10; i.e. the reaction does not go to completion and the yield of the desired product will not be very high.
An alternative method for the preparation of esters is to treat the alcohol with a reactive carboxylic acid derivative, for example the carboxylic acid anhydride or acid chloride. These reactions are effectively irreversible. They are also rapid, particularly when catalysed by strong acids.
(RCO)2O + R'OH
RCOOR' + RCOOH
RCOCl + R'OH
RCOOR' + HCl
Experimental Notes
In the first part of this experiment, you react acetic anhydride with salicylic acid to produce acetylsalicylic acid and acetic acid; sulfuric acid is used as a catalyst. The excess acetic anhydride is then decomposed with water to form acetic acid. Acetylsalicylic acid is not very soluble in water (~ 0.25 g/100 mL). Consequently it can be isolated by diluting the reaction mixture with water and filtering off the solid product.
In the second part, you use thin layer chromatography (TLC) to identify the components of analgesic (pain-killing) tablets as well as confirming the identity and purity of the aspirin synthesized in the first part of the experiment. You will see how TLC can be used to analyse standards of caffeine and three analgesics, aspirin, paracetamol and ibuprofen. If you have previously completed E25, you will also be able to use TLC to examine the purity of the caffeine you extracted in that experiment.