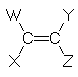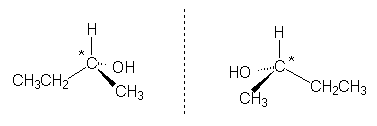Isomerism in organic chemistry
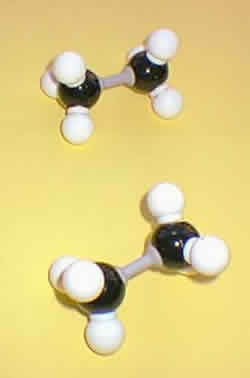
The term isomer is applied to compounds which have identical molecular formulae, but differ in the nature or sequence of bonding and/or in the arrangement of their atoms in space. Models can be used to help understand the differences between the various types of isomers. Molecular shape is a fundamental concept in organic chemistry. Molecules are three-dimensional, and the spatial interactions within or between molecules can be very important in determining a compound's chemical and physical properties.
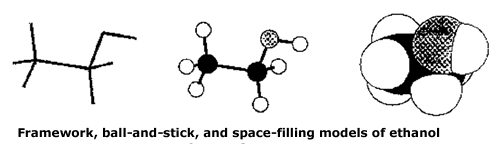
Various types of model sets are available: framework and ball and stick models are useful in visualising many of the three-dimensional aspects of organic structures, while space-filling models, showing the van der Waals radii of atoms, are useful for studying intermolecular interactions (e.g. the interaction of a drug with sites on a protein or DNA molecule) |
||
 |
||
The different types of isomers and how they are related are shown in the following diagram.
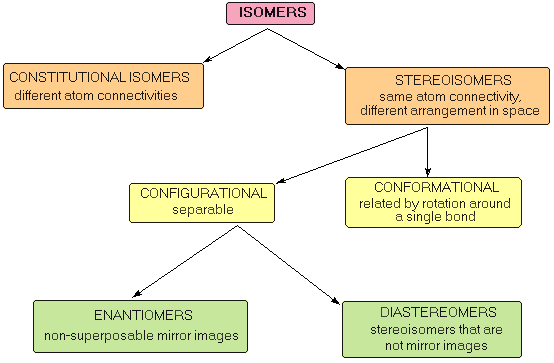 |
Constitutional Isomers
Definition: Constitutional isomers possess different bond connectivities and have different physical and chemical properties.
The constitution of a compound defines the nature and sequence of bonding of the atoms. Isomers differing in constitution are termed constitutional isomers. Ethyl methyl ether and 1-propanol are constitutional isomers.
Stereoisomers
Definition : Stereoisomers are isomers with the same constitution, but differ in the arrangement of their atoms in space. They may have different physical and chemical properties.
Stereoisomers can be defined further into conformers and configurational isomers .
Conformational Isomers
Definition : Conformational isomers (conformers) are structures that differ only by rotation about a single bond. They cannot normally be separated and possess identical physical and chemical properties.
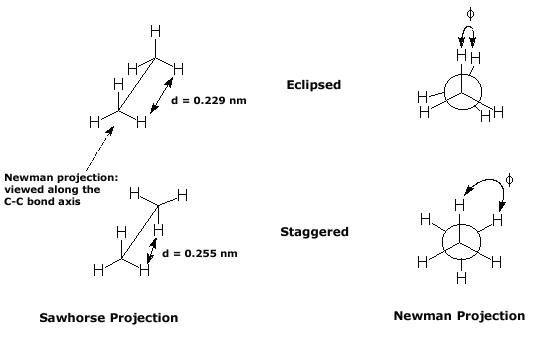
No bonds are broken when one conformation is converted into another. For example, the following structures represent two of the many possible conformational isomers of ethane. These conformational isomers rapidly interconvert at room temperature and individual isomers cannot be isolated. Ethane may be represented in a Newman projection (as opposed to the normal sawhorse drawings) where the molecule is viewed along the bond between the two carbon atoms. The other bonds attached to the front atom are represented by the lines radiating from the centre of a circle. Groups attached to the rear atom are joined by short lines to the circumference of the circle.
 |
|
|
|
||||
Space filling model of sawhorse projection |
Space filling model of Newman projection |
The two conformations illustrated above represent the maximum and minimum energy conformations of ethane (eclipsed and staggered, respectively). In the eclipsed conformation the distance between the hydrogens on adjacent carbon atoms is slightly less than twice the van der Waals radius of hydrogen (2 x 0.12 nm = 0.24 nm), so that steric repulsions occur. Experimentally it has been determined that the eclipsed conformation is about 12 kJ mol-1 higher in energy (i.e. less stable) than the staggered conformation.
An energy level diagram for the conformations of ethane (as given by their dihedral or twist angle) is represented by a sinusoidal curve:
The energy level diagram for the conformations of butane (CH3-CH2-CH2-CH3) is slightly more complicated as there are two possible eclipsed conformations and two
possible staggered conformations for rotation about the central C-C bond:
Configurational Isomers
Definition : Configurational isomers differ in the arrangements of atoms in space, but excludes conformers. To change one configurational isomer into another requires breaking of bonds.
Configurational isomers can be conveniently divided into two classes: enantiomers and diastereoisomers (also called diastereomers).
Diastereoisomers
Definition : Diastereoisomers are configurational isomers that are not mirror images of one another.
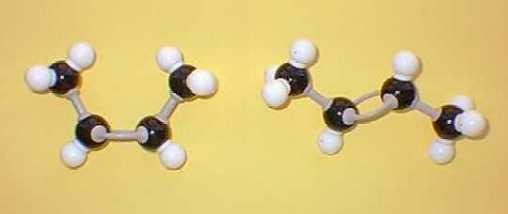
For example, 2-butene has two diastereoisomers which have different physical and chemical properties. The isomers differ in the relative position of the CH3 groups with respect to each other. The two isomers are named according to whether the CH3 groups are on the same side (Z), (from the German zusammen meaning together) or on opposite sides (E), (from the German entgegen meaning opposite) of the double bond. Note that (E) and (Z) are italicised, but may be handwritten underlined: (E) and (Z).
 |
Space filling models look like this:
 |
|
In general, diastereisomers are possible for alkenes of the form:
|
where
|
Sequence Rule
The order of priority is determined by the atomic number of the atoms directly attached to the carbon atom of interest. Those atoms with higher atomic number have priority over those with lower atomic number.
Hence:
I > Br > Cl > OH > NH2> CH2> H.
When the first atom of two (or more) groups is of the same kind, then the next atoms along the chain are considered, and so on until a decision is reached.
Cis and Trans Isomers
This nomenclature is frequently used for naming cyclic compounds and describes the relative position of two substituents with respect to the ring. If a selected pair of groups are on the same side of a reference plane common to both isomers (the ring of a cyclic compound), the groups are said to be cis (handwritten as cis). If the groups are on opposite sides of the common reference plane, they are said to be trans (handwritten as trans).
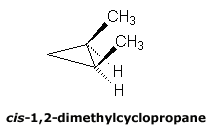 |
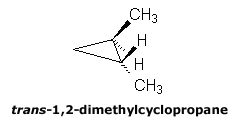 |
 |
|
Enantiomers
Definition : An enantiomer is a configurational isomer that is non-superposable on its mirror image.
A pair of enantiomers are like a left and right hand (i.e. non-superposable mirror images), and are said to be chiral (from the Greek cheir meaning hand). All other molecules are achiral (i.e. without chirality). Many chiral molecules contain a tetrahedrally surrounded central atom (often a carbon atom) bearing four different substituents. Such an atom is known as a stereogenic centre. Molecules can contain more than one stereogenic centre and these may or may not be themselves chiral. Chiral molecules have no plane(s) of symmetry or centre of inversion and it is often easier to try to spot these to prove that a molecule is achiral rather than try to draw and superimpose mirror images.
2-Butanol is an example of a molecule containing one stereogenic centre (marked with an asterisk):
|
||
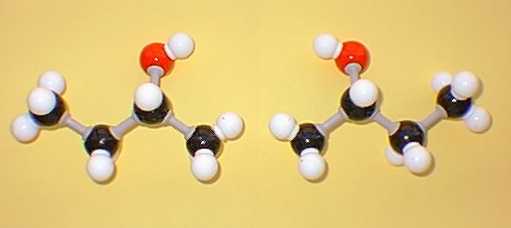 |
||
Nomenclature of Enantiomers
The four atoms or groups a, b, c, d joined to a stereogenic centre are ordered in decreasing priority such that a>b>c>d (see The Sequence Rule above) and the molecule is positioned so that the lowest priority group, d, is remote from the observer who is looking down the C-d bond. If the sequence a --> b --> c is clockwise the molecule is labelled (R) (from the Latin rectus meaning right), and if the sequence a --> b --> c is anticlockwise the molecule is labelled (S) (from the Latin sinister meaning left). The labels (R) and (S) may be handwritten underlined: (R) and (S) respectively.
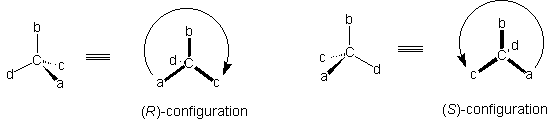 |