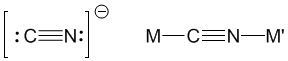Prussian Blue
Molecules and solids with contain large enough holes to trap other molecules, atoms or ions are of considerable interest as nanomaterials. The 'hydrogen economy' relies on being able to safely carry highly explosive H2 around in cars and release it when required. The holes or 'pores' in the molecular framework of some of these nanomaterials are capable of storing small molecules like H2. Others are able to trap unwanted pollutants such as cations of poisonous heavy metals and act as molecular sieves. The School of Chemistry is at the forefront of the development of these materials.
Prussian Blue was the first synthetic colour to be created, way back in the early 1700s. It is now being investigated as a possible nanomaterial for removing Tl+ and Cs+ ions in radioactive material. You have already made Prussian Blue this semester when you mixed potassium hexacyanoferrate(III) with an Fe2+ compound in 'E1 Chemistry with Light' (week 3) and you may remember its beautiful, deep blue colour. You will make it again this week and also make analogues of it with different M2+ metal ions. You will then test the ability of these nanomaterials to trap metal ions.
Prussian Blue itself has the framework structure shown below. Rotate the structure to see the large holes present inside the cubes.
The structure contains Fe2+ and Fe3+ ions. Click on the buttons below to see the position of these ions. In this experiment, you will also make frameworks in which Fe2+ is replaced by other M2+ transition metal ions. These ions are connected together with ligands. In Prussian Blue, the ligand is cyanide (CN-) which is capable of bonding to two metals at once. The framework structure with metal ions on the corners and cyanide spacers leads to large pores in which water molecules normally sit but into which other metal ions can be introduced. | |
| The metal ions that are introduced into the pores are held sufficiently strongly that they are efficiently removed from the surrounding solution. Their absence from the solution following uptake by the framework can be detected using a flame test on the solution. | |
Each Fe2+ and Fe3+ ion is joined to six cyanide ligands so that the structure is made up of many of these cubes, to make an infinite network. |
| The blue colour of Prussian Blue is due to absorption of light. The light causes an electron to transfer from Fe2+ to Fe3+. As discussed in E1, the blue colour we see is the complementary colour of the absorped light. |
Flame Tests
Flame tests are a quick and easy way of detecting the presence (or absence) of certain metal ions.
In the heat of a flame, electrons are excited into orbitals in higher shells. When they 'relax' and return to their original
energy level, they emit light. The energies of an atom's orbitals depend on its nuclear charge and on the
shielding by the other electrons. As a result, each element has a characteristic emission spectrum that can be used to identify it. In part of the emission falls
in the visible region, we see it as a colour. Although the actual wavelengths emitted are unique to each element, our eyes may not be able
to detect small differences so that never every element has a unique colour in a flame. Nevertheless, flame tests are very useful for identifying
certain elements.
The table below shows some common metals and their corresponding flame colours. Hover over the element name to see its flame test colour:
|
| The colour of the flame is due to the emission of light. Electrons are excited by the heat of the flame and emit light when they return to their normal energy. Both the metal ion and the metal atom contribute to the flame colour. The colour we see is the same as that emitted. |