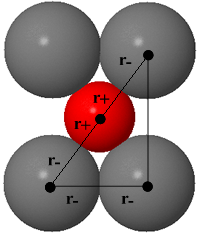
Limiting radius ratio for 8-coordination (caesium chloride structure)
|
The rotatable structure opposite shows the CsCl structure in which the Cs+ ion is surrounded by 8 Cl- ions. To get as close to the cation as possible, the anions must touch along the edge of the cube, as shown in the figure below. Click the 'Directions In Which Anions Touch' button to see this view. The side of the cube has a length, a, where: a = 2r- Along the body diagonal, the Cs+ is touching the two Cl- ions on either end so its length, d, is:d = r- + 2r+ + r- = 2r+ + 2r- |
| |||||
|
||||||
Using Pythagoras’ theorem, the length of the side and the body diagonal of a cube are related:
d2 = a2 + a2 + a2 = 3a2 = 3 × 4r-2 = 12r-2
d = 2(3)1/2r-
2r- + 2r+ = 2r(3)1/2r-
r+ / r- = (3)1/2 – 1 = 0.732
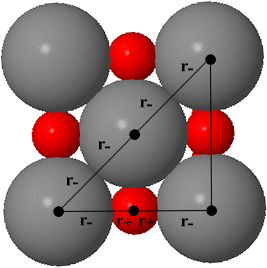
Limiting radius ratio for 6-coordination (sodium chloride structure)
|
The rotatable structure opposite shows the NaCl structure in which the Na+ ion is surrounded by 6 Cl- ions. As shown in the figure below, along the cube edge, the Na+ is touching two Cl- ions so its length, a, is: a = r- + 2r+ + r- = 2r+ + 2r- To get as close to the cation as possible, the anions must touch along the diagonal of a face of the cube. Click the 'Directions In Which Anions Touch' button to see this view. The diagonal has length, d, where:d = r- + 2r- + r- = 4r- |
| |||||
|
||||||
Using Pythagoras’ theorem, the length of the side and face diagonal of a cube are related:
d2 = a2 + a2 = 2a2
d = (2)1/2a
4r- = (2)1/2(2r+ + 2r-)
r+ / r- = (2)1/2 – 1 = 0.414
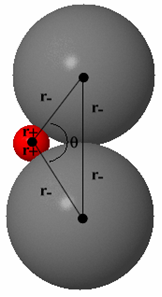
Limiting radius ratio for 4-coordination (zinc blende structure)
|
The rotatable structure opposite shows the ZnS (zinc blende) structure in which the Zn2+ ion is surrounded by 4 S2- ions. The distance from the centre of the tetrahedron to the corner, d, is: d = r+ + r- To get as close to the cation as possible, the anions must touch along the edge of the tetrahedron. Click the 'Directions In Which Anions Touch' button to see this view. This distance, a, isa = r- + r- = 2r- |
| |||||
|
||||||
The tetrahedral angle, q, is 109.5° so
sin(q/2) = r- / (r+ + r-)
So,sin(54.7°) = r- / (r+ + r-)
r+ / r- = 0.225