Electron Transfer Reactions
Electron Transfer
There are many reactions that involve electron transfers. For example, the reaction between Fe3+ and H2S below involves the transfer of electrons from sulfur to iron.
H2S + 2Fe3+
2H+ + S + 2Fe2+
In any such transfer of electrons there is a change in the oxidation number of the species involved. For the example above, iron goes from +III on the left of the equation to +II on the right through a process of gaining electrons; sulfur changes from -II to 0 by a loss of electrons. When the oxidation number of a species becomes more positive in value, then that species is said to have been oxidised. If the oxidation state becomes less positive then the species is said to have been reduced. Thus, in our example, iron has been reduced and sulfur has been oxidised.
The overall reaction above is termed an oxidation/reduction (or redox) reaction and can be broken up into several half-equations that show the two processes of oxidation and reduction in isolation, as shown below. These half-equations are more properly termed ion/electron half-equations.
Fe3+ + e-
Fe2+
H2S 2H+ + S + 2e-
In a redox reaction, the species that causes oxidation of another species is called the oxidant and is itself reduced in the process.
On the other hand, the species that causes reduction in another species is called the reductant, and undergoes oxidation in the process.
In our example, then, iron(III) is the oxidant and hydrogen sulfide is the reductant. Importantly, for a redox reaction to occur there must exist both a simultaneous oxidation and reduction process.
Redox Equations
The process for constructing ion/electron half-equations and combining them to give a redox equation is given below.
Also, see the worked example.
Constructing Half-Equations
- Balance the numbers of all the atoms other than O and H.
- Balance O by adding H2O to either side.
- Balance H by adding H+ to either side.
- Balance the charges by adding electrons to either side.
- If basic conditions are specified: eliminate any H+ present by adding enough OH- to each side of the reaction equation to turn H+ into H2O.
Combining Half-Equations
If two half-equations are to be combined, the electrons must be algebraically eliminated by multiplying each half-equation by a whole number. Take the following example:
Fe3+ + e-
14H+ + Cr2O72- + 6e- 2Cr3+ + 7H2O
In order to combine these two half-equations together, first multiply the iron oxidation half-equation by 6, giving
6Fe3+ + 6e-
Then, when this is added to the dichromate reduction half-equation, cancel anything that appears on both sides of the equation, including the electrons. Hence, the overall redox reaction is
14H+ + Cr2O72- + 6Fe2+ 2Cr3+ + 7H2O + 6Fe3+
Electrochemical Couples
Homogeneous Couples
The combination of the reactant and product in a single ion/electron half-equation is described as an electrochemical couple . If all species are in solution, the couple is described as being homogeneous. An example is a solution containing iron(III) sulfate and iron(II) sulfate, where the couple is represented as Fe3+ / Fe 2+ (in this type of shorthand representation, the oxidised species appears first). In isolation the solution containing the couple is stable, but when it is mixed with a solution containing another couple a redox reaction almost always occurs. One couple moves towards its reduced species, the other towards its oxidised species, until equilibrium is established. Treatment of the above couple with a suitable reductant converts the Fe3+ of the couple into Fe2+, while treatment with a suitable oxidant converts Fe2+ into Fe3+ .
The extent to which these movements occur is determined by two factors:
- The relative reducing/oxidising power of a particular couple
- The relative concentrations of the four solutes.
Heterogeneous Couples
The species that make up a couple need not all be in solution: one member may be a solid, as in Cu2+ (aq) / Cu(s), or a gas, as in H+ (aq) / H2(g). Any such couple that involves transition between species in different states is called a heterogeneous couple.
For instance, when combined with a Cu2+ / Cu couple, a Zn2+ / Zn couple undergoes oxidation. The equation for the reaction is
Cu(s) + Zn2+ (aq)
Electrochemical Cells
When the Cu2+ / Cu couple and Zn2+ / Zn couple are combined as shown in the diagram below, electron-transfer will take place through the conducting wire.
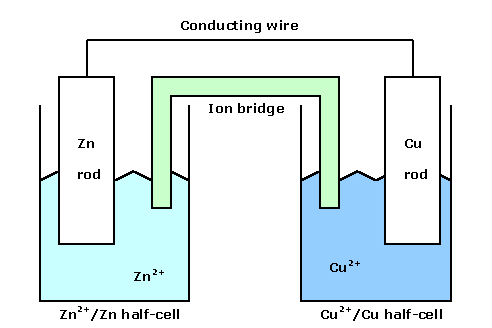 |
This system is called an electrochemical cell. The half-reaction taking place in the Zn2+ / Zn half-cell is
Zn2+ + 2e-
and in the Cu2+ / Cu half-cell is
Cu(s)
Thus the electrons flow through the wire from the Zn rod (or electrode) to the Cu rod. A solution of an electrolyte must always be electrically neutral. Consequently, because positive ions are produced at the surface of the Zn rod and are removed from solution at the surface of the Cu rod, electrical neutrality can only be maintained if ions "flow" between the two half-cells. This flow of ions is provided by the ion bridge, which consists of a solution of electrolyte supported by absorbent paper (or other means). In the cell above, the neutrality is maintained by the movement of positive ions within the ion bridge towards the Cu2+ / Cu half-cell and of negative ions towards the Zn2+ / Zn half-cell. Note that it is the ions within the bridge that provide the balance and not the transfer of ions from one solution to the other.
In this example, the metal electrode which is one component of each couple provides the link between the half-cell and the conducting wire. In a half-cell lacking a metal electrode, such as in a homogeneous couple, the link is provided by a non-reacting solid conductor such as a graphite or platinum electrode. Moreover, a measure of the potential difference between the half-cells can be determined through the inclusion of a voltmeter in the conducting wire.
See Appendix 1.
Experimental Notes
In this exercise, some redox reactions and their applications to cells are investigated. The ion/electron half-reactions corresponding to the following couples are to be ranked in the order of their tendency to undergo reduction. That is, in the order of their electrode potentials.
Br2 / Br- |
I2 / I- |
Fe3+ / Fe2+ |
H+ ,MnO2- / Mn2+ |
Sn4+ / Sn2+ |
In each experiment only one member of each couple being investigated is present initially. This has two consequences:
- Where reaction occurs, this is shown by the appearance of the second member of the couple and/or by the disappearance of the first.
- When the reaction occurs, the second member of each couple is formed.