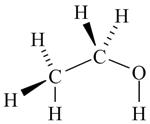
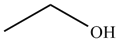
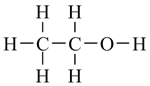The alcohol which some people drink is called ethanol and has the molecular formula C2H6O. Its actual structure can be represented in a number of different ways, each of which has a particular level of sophistication and usefulness.
| 2-dimensional representations | |||||||
|
|
|
|||||
condensed structural formula |
structural formula |
structural formula showing stereochemistry |
stick structure |
||||
|
|
| ||||||
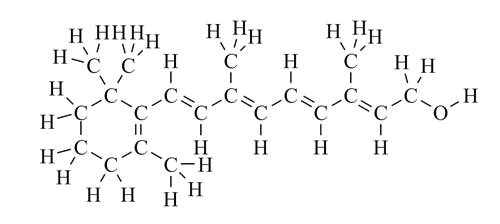
Ethanol is a very simple molecule, but as soon as any complexity is introduced, it is very difficult to comprehend a molecule's structure if all the H atoms are included. So in practice, the stick representation is always used for all but the simplest molecules.
In a stick structure:
- Lines represent bonds - 1, 2 or 3 lines for single, double or triple bonds respectively.
- Carbon atoms are not shown - they are assumed to be at intersections and ends of lines (bonds).
- C–H bonds are omitted - the number of hydrogens bonded to each carbon can be calculated, as carbon always has a valence of 4.
- All heteroatoms are shown and so are the hydrogens bonded to them.
- When drawing neutral molecules: C always has valence of 4 (4 bonds, 0 lone pairs) N always has valence of 3 (3 bonds, 1 lone pair) O always has valence of 2 (2 bonds, 2 lone pairs) F, Cl, Br, I always have valence of 1 (1 bond, 3 lone pairs).
- The bond angles around carbons, nitrogens and oxygens are usually drawn as 120° or 90°, though the actual bond angle is closer to 109.5° unless double or triple bonds are involved. Otherwise, bond angles are drawn to be as realistic as possible.
- Some aspects of stereochemistry may be shown if relevant to the discussion at hand.
|
|
| ||