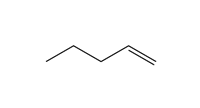
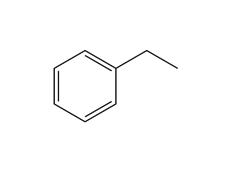
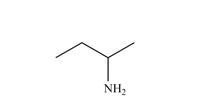
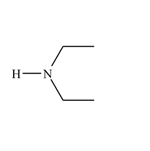
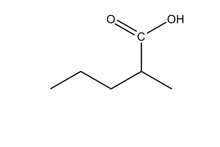
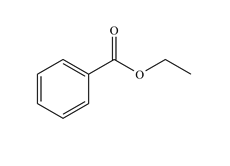
A functional group is a group of atoms that has a characteristic chemical behaviour. An organic molecule can be viewed as an inert skeleton of C–C single bonds saturated with H's with functional groups attached at different positions. It is the functional groups that determine the types of reactions that the molecule will undergo. Functional groups are as basic to organic chemistry as words are to language or chords are to music. When you take an organic course, they need to be learnt and committed to memory as soon as possible - understanding organic chemistry is impossible if this is not done. To help you to memorize them, try out the:
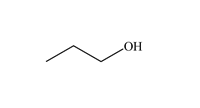
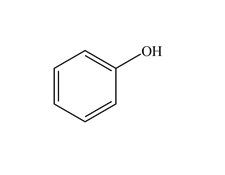
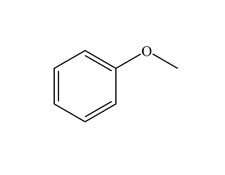
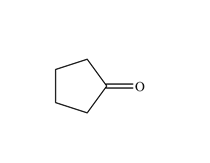
Functional Group FlashcardsThere are many functional groups, some very common, others less so. The table below lists some common function groups. The structures have been drawn as stick structures - roll the mouse over the molecule to reveal the full structural representation. For help on drawing stick structures, see the pages on organic structures and stick structures and formulae.